
Prof. Karl-Josef DIETZ
Bielefeld University
Since 1997 the chair of Plant Biochemistry and Physiology is held by Prof. Karl-Josef Dietz, following Prof. Wolfgang Kowallik. The Dietz lab is a member of the Faculty of Biology and is integrated into the CeBiTec (Center for Biotechnology) at Bielefeld University as part of the Institute for Genome Research and Systems Biology (IGS).
Using up-to-date methods of proteomics, transcriptomics incl. translatome analysis, dynamic cell imaging, molecular biology and recombinant protein analyses, we aim to decipher the molecular and physiological mechanisms of damage development and efficient stress acclimation. To this end, we investigate e.g. high light acclimation, salinity and heat stress in Arabidopsis, sugar beet and rice, and pharmacological impact in plant and human cell models.
https://www.uni-bielefeld.de/fakultaeten/biologie/forschung/arbeitsgruppen/plant_biochem/index.xml
Molecular Editing of 12-Oxophytodienoic Acid for Functional Analysis and Differential Tuning of Oxylipin Signaling in Planta
Madita Kniepera, Maike Bittmannb, Ruben Schwarza, Andrea Viehhausera, Harald Grögerb, Karl-Josef Dietza*
a Biochemistry and Physiology of Plants, Bielefeld University, Germany
b Industrial Organic Chemistry and Biotechnology, Bielefeld University, Germany
*karl-josef.dietz@uni-bielefeld.de
Oxylipins are oxidation products of polyunsaturated fatty acids that regulate physiological processes in plants and animals.1 Prostaglandins in animals and 12-oxophytodienoic acid (OPDA) and jasmonic acid in plants, are such oxylipins that play roles e.g. in wound responses, reproduction and environmental acclimatization. OPDA is synthesized from α-linolenic acid in the chloroplast by consecutive action of three enzymes (13-lipoxygenase [13-LOX], allene oxide synthase, allene oxide cyclase). In addition to its function as metabolic precursor of the plant hormone jasmonic acid, OPDA is a versatile regulator of diverse biological processes including chloroplast cysteine synthesis, protein activity regulation by OPDAylation through Michael addition, reaction with other free thiols and inhibitor of 13-LOX1,2. These functions can partly be addressed genetically with drawbacks by pleiotropic effects and broad disturbance of plant physiology, or pharmacologically. However, at the start of the project, OPDA was available only at low amounts and high prices.
We first established a bio-organic route for synthesizing OPDA3 and this process was optimized and upscaled4,5. However, the challenge was to obtain derivatives for scrutinizing the different physiological roles of OPDA, and this was realized by olefin-cross metathesis using a Hoveyda-Grubbs 2nd generation catalyst5. The outcome is a library of 15 molecularly edited OPDA derivatives for functional analysis in vitro and in vivo. It will be shown that this library is a unique tool to dissect the various functions of OPDA. An example is the differential binding to target proteins like cyclophilin 20-3 to activate chloroplast cysteine synthesis, the altered activity as Michael acceptor to glutathione or different triggering of stomatal closure in planta.6 Successful molecular editing of OPDA for functional scrutiny in vivo may serve as blueprint for similar approaches in biology and medicine.
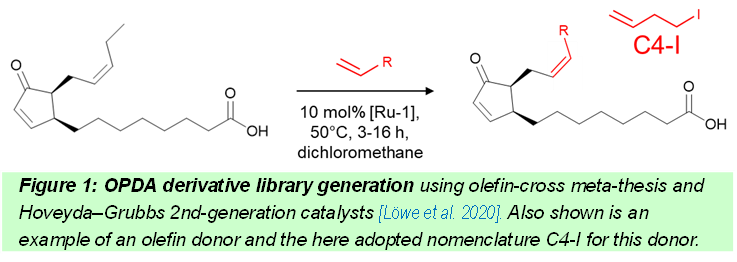
References:
1. Maynard, D. et al., J. Exp. Bot. 2018, 69 (22), 5341-5354
2. Knieper M. et al., bioRxiv, 2025, DOI 10.1101/2025.07.31.667886
3. Maynard, D. et al., Bioorg. Medicinal Chem. 2018, 26, 1356-1364
4. Guntelmann, T.L., Dietz, K.J., Gröger, H., Org. Biomol. Chem. 2024, 202422, 5406-5413
5. Löwe, J., Dietz, K.J., Gröger H, Adv. Science 2020, 7, 1902973
6. unpublished


